如果你也在 怎样代写无机化学inorganic chemistry这个学科遇到相关的难题,请随时右上角联系我们的24/7代写客服。无机化学inorganic chemistry涉及到无机和有机金属化合物的合成和行为。这个领域涵盖了非碳基的化合物,这些化合物是有机化学的主题。这两门学科之间的区别远非绝对,因为有机金属化学的分支学科有很多重叠。它在化学工业的各个方面都有应用,包括催化、材料科学、颜料、表面活性剂、涂料、药物、燃料和农业。
无机化学inorganic chemistry许多无机化合物是离子化合物,由阳离子和阴离子通过离子键连接组成。盐(属于离子化合物)的例子有氯化镁MgCl2,它由镁的阳离子Mg2+和氯的阴离子Cl-组成;或氧化钠Na2O,它由钠的阳离子Na+和氧化阴离子O2-组成。在任何盐中,离子的比例是这样的:电荷相互抵消,因此大部分化合物是电中性的。离子由其氧化状态描述,其形成的难易程度可以从电离电位(阳离子)或从母元素的电子亲和力(阴离子)推断出来。
my-assignmentexpert™ 无机化学inorganic chemistry作业代写,免费提交作业要求, 满意后付款,成绩80\%以下全额退款,安全省心无顾虑。专业硕 博写手团队,所有订单可靠准时,保证 100% 原创。my-assignmentexpert™, 最高质量的无机化学inorganic chemistry作业代写,服务覆盖北美、欧洲、澳洲等 国家。 在代写价格方面,考虑到同学们的经济条件,在保障代写质量的前提下,我们为客户提供最合理的价格。 由于统计Statistics作业种类很多,同时其中的大部分作业在字数上都没有具体要求,因此无机化学inorganic chemistry作业代写的价格不固定。通常在经济学专家查看完作业要求之后会给出报价。作业难度和截止日期对价格也有很大的影响。
想知道您作业确定的价格吗? 免费下单以相关学科的专家能了解具体的要求之后在1-3个小时就提出价格。专家的 报价比上列的价格能便宜好几倍。
my-assignmentexpert™ 为您的留学生涯保驾护航 在化学Chemical作业代写方面已经树立了自己的口碑, 保证靠谱, 高质且原创的无机化学inorganic chemistry代写服务。我们的专家在化学Chemical代写方面经验极为丰富,各种无机化学inorganic chemistry相关的作业也就用不着 说。
我们提供的无机化学inorganic chemistry及其相关学科的代写,服务范围广, 其中包括但不限于:
化学代写|无机化学作业代写INORGANIC CHEMISTRY代考|Ozone molecule (O3)
There are six valence cell electrons over central ‘ $O$ ‘ considering only $\sigma$-bond formation (excluding $\pi$ electrons). So, the structure of ozone molecule is triangular planar structure with one lp over central ‘ $O$ ‘ (Figure 2.1).
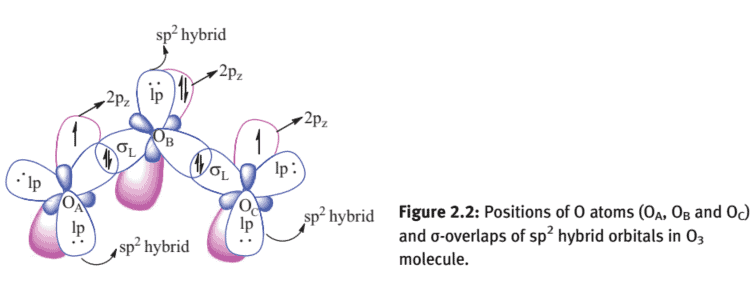
$\mathrm{O}{3}$ forms a $\mathrm{V}$-shaped molecule. Both the bond lengths are $1.278 \AA$, and the bond angle is $116^{\circ} 48^{\prime}$. It may be assumed that the central $\mathrm{O}$ atom $\left(\mathrm{O}{\mathrm{B}}\right)$ uses roughly sp ${ }^{2}$ hybrid orbitals for $\sigma$ bonding. The same may be considered for the other two oxygens, $\mathrm{O}{\mathrm{A}}$ and $\mathrm{O}{\mathrm{C}}$ (Figure 2.2).
The presence of delocalized $\pi$-bonding in the $\mathrm{O}{3}$ molecule is best explained by delocalized three-centred $\pi$-bonding as follows: (i) There is a total of $18 \mathrm{e}$ in the valence shell considering six electrons from each of the three $O$-atoms $\left(2 s^{2} 2 p^{4}\right)$. (ii) The central oxygen atom $\left(\mathrm{O}{\mathrm{B}}\right)$ forms a $\sigma$-bond with two end oxygens, $\mathrm{O}{\mathrm{A}}$ and $\mathrm{O}{\mathrm{C}}$, which accounts for $4 \mathrm{e}$. As shown in the hybridization given in Figures $2.3$ and $2.2$, the central $\mathrm{O}$ atom $\left(\mathrm{O}_{\mathrm{B}}\right)$ uses two of its singly filled $\mathrm{sp}^{2}$ hybrid orbitals, and the remaining one is having a lp.
化学代写|无机化学作业代写inorganic chemistry代考|Nitrogen dioxide (NO2) molecule
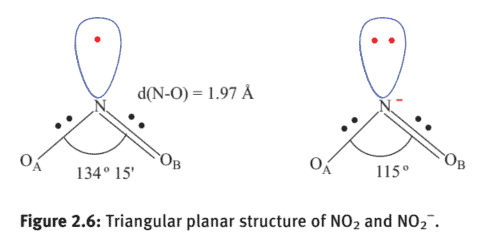
This is an odd electron molecule in terms of VBT, which can be represented as shown in Figure 2.6. The number of valence shell electrons surrounding the central $\mathrm{N}$ considering only $\sigma$ bonding is five ( $2.5$ electron pairs, that is, $2 \mathrm{bp}+1$ lone electron). Based on the VSEPR theory, the geometry of the molecule is triangular planar. The $\mathrm{O}-\mathrm{N}-\mathrm{O}$ bond angle is $134^{\circ} 15^{\prime}$ because of smaller effect in contraction of bond angle due to a lone electron than the lone pair as in $\mathrm{NO}_{2}^{-}(3$ electron pairs; $2 \mathrm{bp}+1 \mathrm{lp})$.
The bond angle suggests that the $\mathrm{N}$ atom of the $\mathrm{NO}{2}$ molecule utilizes sp hybrid orbitals for $\sigma$ bonding (Figure 2.7) leaving $2 \mathrm{p}{\mathrm{z}}$ orbitals free to involve in $\pi$-overlap. A similar hybridization may be considered for the other two oxygen atoms $\mathrm{O}{\mathrm{A}}$ and $\mathrm{O}{\mathrm{B}}$. Positions of the atoms and $\sigma$-overlaps have been shown in Figure 2.7. The hybridization scheme (Figure 2.8) also makes it clear.
化学代写|无机化学作业代写INORGANIC CHEMISTRY代考|Nitrite ion (NO2− )
The number of valence shell electrons surrounding the central $\mathrm{N}$ considering only $\sigma$ bonding is six ( 3 electron pairs, i.e. $2 \mathrm{bp}+1 \mathrm{lp}$ ). Based on the VSEPR theory, the geometry of the molecule is triangular planar (Figure 2.11). The ONO bond angle is $115^{\circ}$ because of greater effect in contraction of bond angle due to a lone pair of electron in $\mathrm{NO}{2}{ }^{-}$compared to the lone electron in $\mathrm{NO}{2}$.
The bond angle suggests that the $\mathrm{N}$ atom of the $\mathrm{NO}{2}{ }^{-}$molecule utilizes $\mathrm{sp}^{2}$ hybrid orbitals for $\sigma$ bonding (Figure $2.12$ ) leaving $2 \mathrm{p}{\mathrm{z}}$ orbitals free to involve in $\pi$-overlap. A similar hybridization may be considered for the other two oxygen atoms $\mathrm{O}{\mathrm{A}}$ and $\mathrm{O}{\mathrm{B}}$. Positions of the atoms and $\sigma$-overlaps have been shown in Figure $2.12$. The hybridization scheme (Figure 2.13) also makes it clear.
无机化学代写
化学代写|无机化学作业代写INORGANIC CHEMISTRY代考|OZONE MOLECULE这3
有六个价电池电子在中心 ‘这’ 只考虑σ- 键形成和XC一世你d一世nG$圆周率$和一世和C吨r这ns. 所以,臭氧分子的结构是三角形平面结构,中心有一个lp’这‘F一世G你r和2.1.
$\mathrm{O}{3}$ forms a $\mathrm{V}$-shaped molecule. Both the bond lengths are $1.278 \AA$, and the bond angle is $116^{\circ} 48^{\prime}$. It may be assumed that the central $\mathrm{O}$ atom $\left(\mathrm{O}{\mathrm{B}}\right)$ uses roughly sp ${ }^{2}$ hybrid orbitals for $\sigma$ bonding. The same may be considered for the other two oxygens, $\mathrm{O}{\mathrm{A}}$ and $\mathrm{O}{\mathrm{C}}$ (Figure 2.2).
离域的存在圆周率-结合在$\pi$-bonding in the $\mathrm{O}{3}$ molecule is best explained by delocalized three-centred $\pi$-bonding as follows: (i) There is a total of $18 \mathrm{e}$ in the valence shell considering six electrons from each of the three $O$-atoms $\left(2 s^{2} 2 p^{4}\right)$. (ii) The central oxygen atom $\left(\mathrm{O}{\mathrm{B}}\right)$ forms a $\sigma$-bond with two end oxygens, $\mathrm{O}{\mathrm{A}}$ and $\mathrm{O}{\mathrm{C}}$, which accounts for $4 \mathrm{e}$. As shown in the hybridization given in Figures $2.3$ and $2.2$, the central $\mathrm{O}$ atom $\left(\mathrm{O}_{\mathrm{B}}\right)$ uses two of its singly filled $\mathrm{sp}^{2}$混合轨道,剩下的有一个 lp。
化学代写|无机化学作业代写INORGANIC CHEMISTRY代考|NITROGEN DIOXIDEñ这2分子
这是一个用 VBT 表示的奇数电子分子,如图 2.6 所示。围绕中心的价层电子数ñ只考虑σ粘合是五$2.5$和一世和C吨r这np一种一世rs,吨H一种吨一世s,$2bp+1$一世这n和和一世和C吨r这n. 基于 VSEPR 理论,分子的几何形状是三角形平面。这这−ñ−这键角为134∘15′因为孤电子对键角收缩的影响小于孤电子对,如ñ这2−(3电子对;2bp+1一世p).
键角表明$\mathrm{N}$ atom of the $\mathrm{NO}{2}$ molecule utilizes sp hybrid orbitals for $\sigma$ bonding (Figure 2.7) leaving $2 \mathrm{p}{\mathrm{z}}$ orbitals free to involve in $\pi$-overlap. A similar hybridization may be considered for the other two oxygen atoms $\mathrm{O}{\mathrm{A}}$ and $\mathrm{O}{\mathrm{B}}$. Positions of the atoms and $\sigma$-如图 2.7 所示。杂交方案F一世G你r和2.8也说清楚了。
化学代写|无机化学作业代写INORGANIC CHEMISTRY代考|NITRITE IONñ这2−
围绕中心的价层电子数ñ只考虑σ粘合是六3和一世和C吨r这np一种一世rs,一世.和.$2bp+1一世p$. 基于 VSEPR 理论,分子的几何形状是三角形平面F一世G你r和2.11. ONO 键角为115∘因为 $\mathrm{NO} {2}{ }^{-}中的孤对电子对键角收缩的影响更大C这米p一种r和d吨这吨H和一世这n和和一世和C吨r这n一世n\mathrm{否} {2}$。
键角表明$\mathrm{N}$ atom of the $\mathrm{NO}{2}{ }^{-}$molecule utilizes $\mathrm{sp}^{2}$ hybrid orbitals for $\sigma$ bonding (Figure $2.12$ ) leaving $2 \mathrm{p}{\mathrm{z}}$ orbitals free to involve in $\pi$-overlap. A similar hybridization may be considered for the other two oxygen atoms $\mathrm{O}{\mathrm{A}}$ and $\mathrm{O}{\mathrm{B}}$. Positions of the atoms and $\sigma$-overlaps have been shown in Figure $2.12$. The hybridization scheme (Figure 2.13) also makes it clear.2.12 美元。杂交方案F一世G你r和2.13也说清楚了。
物化学代写|无机化学作业代写inorganic chemistry代考 请认准UprivateTA™. UprivateTA™为您的留学生涯保驾护航。