如果你也在 怎样代写材料物理Materials Physics这个学科遇到相关的难题,请随时右上角联系我们的24/7代写客服。材料物理Materials Physics是利用物理学来描述材料的物理特性。它是物理科学的综合,如化学、固体力学、固态物理学和材料科学。材料物理学被认为是凝聚态物理学的一个子集,并将基本的凝聚态概念应用于复杂的多相介质,包括具有技术意义的材料。
材料物理Materials Physics从事的领域包括电子、光学和磁性材料、新型材料和结构、材料中的量子现象、非平衡物理学和软凝聚态物理学。新的实验和计算工具正在不断改善材料系统的建模和研究方式,也是材料物理学家工作的领域。
my-assignmentexpert™ 材料物理Materials Physics作业代写,免费提交作业要求, 满意后付款,成绩80\%以下全额退款,安全省心无顾虑。专业硕 博写手团队,所有订单可靠准时,保证 100% 原创。my-assignmentexpert™, 最高质量的材料物理Materials Physics作业代写,服务覆盖北美、欧洲、澳洲等 国家。 在代写价格方面,考虑到同学们的经济条件,在保障代写质量的前提下,我们为客户提供最合理的价格。 由于统计Statistics作业种类很多,同时其中的大部分作业在字数上都没有具体要求,因此材料物理Materials Physics作业代写的价格不固定。通常在经济学专家查看完作业要求之后会给出报价。作业难度和截止日期对价格也有很大的影响。
想知道您作业确定的价格吗? 免费下单以相关学科的专家能了解具体的要求之后在1-3个小时就提出价格。专家的 报价比上列的价格能便宜好几倍。
my-assignmentexpert™ 为您的留学生涯保驾护航 在物理physics作业代写方面已经树立了自己的口碑, 保证靠谱, 高质且原创的材料物理Materials Physics代写服务。我们的专家在物理physics代写方面经验极为丰富,各种材料物理Materials Physics相关的作业也就用不着 说。
我们提供的材料物理Materials Physics及其相关学科的代写,服务范围广, 其中包括但不限于:
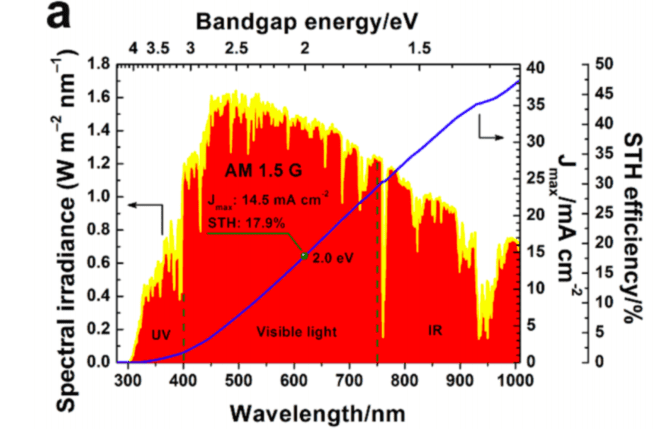
物理代写|材料物理作业代写Materials Physics代考|Energetics of water splitting
Cathode: $\quad 2 \mathrm{H}^{+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \mathrm{H}{2}(\mathrm{~g}) \quad \varepsilon^{0}=0 \mathrm{~V}$ vs NHE $(\mathrm{pH}=0)$ Anode: $\quad 2 \mathrm{H}{2} \mathrm{O}(\mathrm{I}) \rightarrow \mathrm{O}{2}(\mathrm{~g})+4 \mathrm{H}^{+}(\mathrm{aq})+4 \mathrm{e}^{-} \quad \varepsilon^{0}=1.23 \mathrm{~V}$ vs NHE $(\mathrm{pH}=0)$ Total reaction: $2 \mathrm{H}{2} \mathrm{O}(\mathrm{I}) \rightarrow 2 \mathrm{H}{2}(\mathrm{~g})+\mathrm{O}{2}(\mathrm{~g})$
Reduction free energy cathode vs NHE $=-z \varepsilon^{0}=0 \mathrm{eV}$
Oxidation free energy anode vs NHE $=z \varepsilon^{0}=4 * 1.23=4.92 \mathrm{eV}$
Total reaction free energy $=0+4.92=4.92 \mathrm{eV}$
$\rightarrow$ We need to supply $4.92 \mathrm{eV}$ of energy to produce 2 molecules of $\mathrm{H}{2}$ and 1 molecule of $\mathrm{O}{2}$ from 2 water molecules
$\rightarrow$ This energy must come from photons!
$\rightarrow$ Thermodynamic requirement $=4$ photons of $1.23 \mathrm{eV}=1008 \mathrm{~nm}$
$\rightarrow$ Additional energy bias (“overpotential”) required to make reaction happen in finite time to overcome kinetic barriers $\approx 0.5 \mathrm{eV}$
$\rightarrow$ Energy losses $\approx 0.3 \mathrm{eV}$
$\rightarrow$ Photoanode with bandgap $\approx 1.23+0.5+0.3 \approx 2.0 \mathrm{eV}$ suitable (assuming single photosystem)
物理代写|材料物理作业代写Materials Physics代考|Energetics of CO2 reduction
For fuel = methane $\left(\mathrm{CH}{4}\right)$ : Cathode: $\quad \mathrm{CO}{2}(\mathrm{~g})+8 \mathrm{H}^{+}(\mathrm{aq})+8 \mathrm{e}^{-} \rightarrow \mathrm{CH}{4}(\mathrm{~g})+2 \mathrm{H}{2} \mathrm{O}(\mathrm{I}) \quad \varepsilon^{0}=-0.24 \mathrm{~V}$ vs NHE $(\mathrm{pH}=7)$
Anode: $\quad 2 \mathrm{H}{2} \mathrm{O}(\mathrm{I}) \rightarrow \mathrm{O}{2}(\mathrm{~g})+4 \mathrm{H}^{+}(\mathrm{aq})+4 \mathrm{e}^{-} \quad \varepsilon^{0}=0.82 \mathrm{~V}$ vs NHE $(\mathrm{pH}=7)$
Total reaction: $\mathrm{CO}{2}(\mathrm{~g})+2 \mathrm{H}{2} \mathrm{O}(\mathrm{l}) \rightarrow \mathrm{CH}{4}(\mathrm{~g})+2 \mathrm{O}{2}(\mathrm{~g})$
Reduction free energy cathode vs NHE $=-z \varepsilon^{0}=-8 *(-0.24)=1.92 \mathrm{eV}$
Oxidation free energy anode vs NHE $=z \varepsilon^{0}=4 * 0.82=3.28 \mathrm{eV}$
Total reaction free energy $=1.92+2 * 3.28=8.48 \mathrm{eV}$
$\rightarrow$ We need to supply $8.48 \mathrm{eV}$ of energy to produce 1 molecule of $\mathrm{CH}{4}$ and 2 molecules of $\mathrm{O}{2}$ from $1 \mathrm{CO}_{2}$ and 2 water molecules
$\rightarrow$ This energy must come from photons!
$\rightarrow$ Thermodynamic requirement $=8$ photons of $1.06 \mathrm{eV}=1170 \mathrm{~nm}$
$\rightarrow$ Cathodic overpotential $\approx 0.5 \mathrm{eV}$, anodic overpotential $\approx 0.5 \mathrm{eV}$
$\rightarrow$ Energy losses $\approx 0.3 \mathrm{eV}$
$\rightarrow$ Photoelectrode with bandgap $\approx 1.06+0.5+0.5+0.3 \approx 2.3 \mathrm{eV}$ required (assuming single photosystem)
物理代写|材料物理作业代写MATERIALS PHYSICS代考|CO2 reduction catalysts
- all-inorganic metal catalysts
e.g. $\mathrm{Cu}, \mathrm{Fe}, \mathrm{Pb}, \mathrm{Ni}$, noble metals (Au, $\mathrm{Ag}, \mathrm{Pd}, \mathrm{Rh}, \mathrm{Ru}$; $\mathrm{Pt}$ less suitable favours $\mathrm{H}{2}$ formation) loaded on electrode as nanoparticles pros: multi-electron reductions, sometimes yield longer alkanes $\left(C{2}-C_{9}\right)$ cons: poor selectivity, get poisoned - organic molecules e.g. pyridinium or tetra-alkyl ammonium cations, aromatic nitrils and esters pros: metal-free, inexpensive, product selectivity, low overpotential
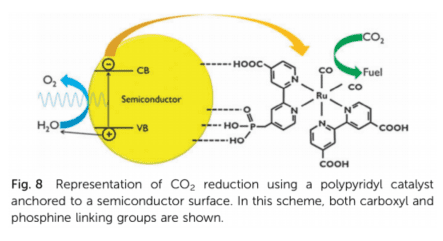
- metal-organic complexes
Ni, Co or Fe-porphyrine, Pd-phospin or Ru-poly-pyridyl complex attached to electrode by covalent bonds pros: improve product selectivity and lower overpotential cons: low stability, low turn-over-frequency - enzymes (biological catalysts)
材料物理代写
物理代写|材料物理作业代写MATERIALS PHYSICS代考|ENERGETICS OF WATER SPLITTING
阴极:$\quad 2 \mathrm{H}^{+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \mathrm{H}{2}(\mathrm{~g}) \quad \varepsilon^{0}=0 \mathrm{~V}$ vs NHE $(\mathrm{pH}=0)$ Anode: $\quad 2 \mathrm{H}{2} \mathrm{O}(\mathrm{I}) \rightarrow \mathrm{O}{2}(\mathrm{~g})+4 \mathrm{H}^{+}(\mathrm{aq})+4 \mathrm{e}^{-} \quad \varepsilon^{0}=1.23 \mathrm{~V}$ vs NHE $(\mathrm{pH}=0)$ Total reaction: $2 \mathrm{H}{2} \mathrm{O}(\mathrm{I}) \rightarrow 2 \mathrm{H}{2}(\mathrm{~g})+\mathrm{O}{2}(\mathrm{~g})$
Reduction free energy cathode vs NHE $=-z \varepsilon^{0}=0 \mathrm{eV}$
Oxidation free energy anode vs NHE $=z \varepsilon^{0}=4 * 1.23=4.92 \mathrm{eV}$
Total reaction free energy $=0+4.92=4.92 \mathrm{eV}$
→我们需要供应4.92和五产生 2 个 $\mathrm{H}{2}$ and 1 molecule of $\mathrm{O}{2}$ from 2 water molecules
$\rightarrow$ This energy must come from photons!
$\rightarrow$ Thermodynamic requirement $=4$ photons of $1.23 \mathrm{eV}=1008 \mathrm{~nm}$
$\rightarrow$ Additional energy bias (“overpotential”) required to make reaction happen in finite time to overcome kinetic barriers $\approx 0.5 \mathrm{eV}$
$\rightarrow$ Energy losses $\approx 0.3 \mathrm{eV}$
$\rightarrow$ Photoanode with bandgap $\approx 1.23+0.5+0.3 \approx 2.0 \mathrm{eV}$ suitable (assuming single photosystem)
物理代写|材料物理作业代写MATERIALS PHYSICS代考|ENERGETICS OF CO2 REDUCTION
对于燃料 = 甲烷 $\left(\mathrm{CH}{4}\right)$ : Cathode: $\quad \mathrm{CO}{2}(\mathrm{~g})+8 \mathrm{H}^{+}(\mathrm{aq})+8 \mathrm{e}^{-} \rightarrow \mathrm{CH}{4}(\mathrm{~g})+2 \mathrm{H}{2} \mathrm{O}(\mathrm{I}) \quad \varepsilon^{0}=-0.24 \mathrm{~V}$ vs NHE $(\mathrm{pH}=7)$
Anode: $\quad 2 \mathrm{H}{2} \mathrm{O}(\mathrm{I}) \rightarrow \mathrm{O}{2}(\mathrm{~g})+4 \mathrm{H}^{+}(\mathrm{aq})+4 \mathrm{e}^{-} \quad \varepsilon^{0}=0.82 \mathrm{~V}$ vs NHE $(\mathrm{pH}=7)$
Total reaction: $\mathrm{CO}{2}(\mathrm{~g})+2 \mathrm{H}{2} \mathrm{O}(\mathrm{l}) \rightarrow \mathrm{CH}{4}(\mathrm{~g})+2 \mathrm{O}{2}(\mathrm{~g})$
Reduction free energy cathode vs NHE $=-z \varepsilon^{0}=-8 *(-0.24)=1.92 \mathrm{eV}$
Oxidation free energy anode vs NHE $=z \varepsilon^{0}=4 * 0.82=3.28 \mathrm{eV}$
Total reaction free energy $=1.92+2 * 3.28=8.48 \mathrm{eV}$
→我们需要供应8.48和五产生 1 个 $\mathrm{CH}{4}$ and 2 molecules of $\mathrm{O}{2}$ from $1 \mathrm{CO}_{2}$ and 2 water molecules
$\rightarrow$ This energy must come from photons!
$\rightarrow$ Thermodynamic requirement $=8$ photons of $1.06 \mathrm{eV}=1170 \mathrm{~nm}$
$\rightarrow$ Cathodic overpotential $\approx 0.5 \mathrm{eV}$, anodic overpotential $\approx 0.5 \mathrm{eV}$
$\rightarrow$ Energy losses $\approx 0.3 \mathrm{eV}$
$\rightarrow$ Photoelectrode with bandgap $\approx 1.06+0.5+0.5+0.3 \approx 2.3 \mathrm{eV}$ required (assuming single photosystem)
物理代写|材料物理作业代写MATERIALS PHYSICS代考|CO2 REDUCTION CATALYSTS
- 全无机金属催化剂,
例如C你,F和,磷b,ñ一世, 贵金属 $\mathrm{Cu}, \mathrm{Fe}, \mathrm{Pb}, \mathrm{Ni}$, noble metals (Au, $\mathrm{Ag}, \mathrm{Pd}, \mathrm{Rh}, \mathrm{Ru}$; $\mathrm{Pt}$ less suitable favours $\mathrm{H}{2}$ formation) loaded on electrode as nanoparticles pros: multi-electron reductions, sometimes yield longer alkanes $\缺点:选择性差,中毒 - 有机分子,例如吡啶鎓或四烷基铵阳离子、芳香腈和酯类 优点:不含金属、价格低廉、产品选择性好、过电位低
- 金属-有机配合物
Ni、Co 或 Fe-卟啉、Pd-膦或 Ru-聚吡啶配合物通过共价键连接到电极上 优点:提高产品选择性和降低过电位 缺点:稳定性低、转换频率低 - 酶(生物催化剂)
物理代写|材料物理作业代写Materials Physics代考 请认准UprivateTA™. UprivateTA™为您的留学生涯保驾护航。