MY-ASSIGNMENTEXPERT™可以为您提供 sydney AMME2262 Thermodynamics热力学课程的代写代考和辅导服务!
这是挪威科技大学 热力学课程的代写成功案例。

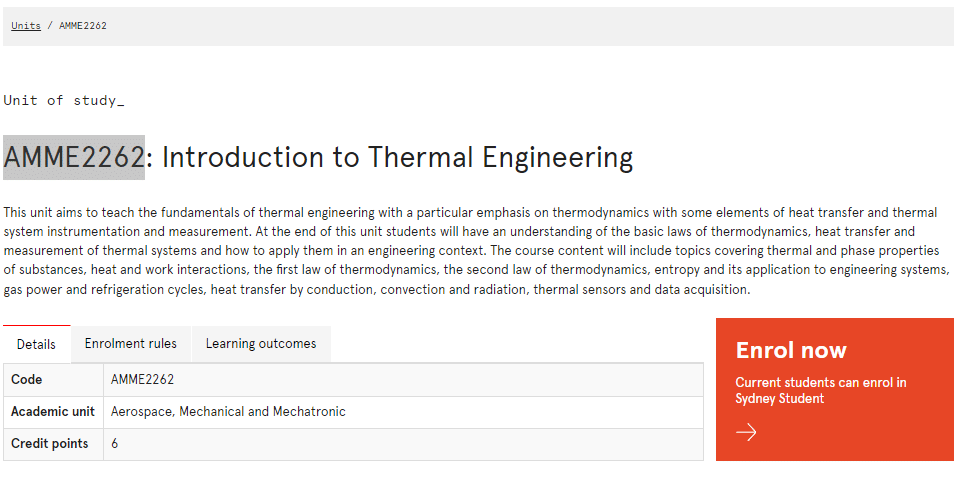
AMME2262课程简介
This unit aims to teach the fundamentals of thermal engineering with a particular emphasis on thermodynamics with some elements of heat transfer and thermal system instrumentation and measurement. At the end of this unit students will have an understanding of the basic laws of thermodynamics, heat transfer and measurement of thermal systems and how to apply them in an engineering context. The course content will include topics covering thermal and phase properties of substances, heat and work interactions, the first law of thermodynamics, the second law of thermodynamics, entropy and its application to engineering systems, gas power and refrigeration cycles, heat transfer by conduction, convection and radiation, thermal sensors and data acquisition.
Prerequisites
At the completion of this unit, you should be able to:
- LO1. write a formal laboratory report
- LO2. work in groups to complete laboratory experiments and analyse the results
- LO3. evaluate the relevant parameters for heat flow in internal engineering systems such as refrigeration and heating systems
- LO4. demonstrate an understanding of some of the basic equations governing thermodynamics
- LO5. analyze the thermodynamics of a simple open or closed engineering system
- LO6. analyze and determine the heat fluxes governing a control volume.
AMME2262 Thermodynamics HELP(EXAM HELP, ONLINE TUTOR)
Free energy of a simple harmonic oscillator
A simple harmonic oscillator has energies $\epsilon_n=n \hbar \omega$, where $n=0,1,2, \cdots$ (neglecting the zeropoint energy of $(1 / 2) \hbar \omega$ which is not important here).
(a) Show that the free energy is equal to
$$
F=k_B T \log \left[1-\exp \left(-\hbar \omega / k_B T\right)\right] .
$$
(b) From this expression, show that the entropy is given by
$$
\sigma \equiv \frac{S}{k_B}=\frac{\left(\hbar \omega / k_B T\right)}{\exp \left(\hbar \omega / k_B T\right)-1}-\log \left[1-\exp \left(-\hbar \omega / k_B T\right)\right] .
$$
There are plots of the entropy and specific heat in Kittel and Kroemer, Figs. 3.13 and 3.14.
For a simple harmonic oscillator the energy levels are
$$
\epsilon_n=n \hbar \omega,
$$
in which, following the question we have set the zero of energy to be at $\epsilon_0$. (More conventionally the energy levels are written $\left.\epsilon_n=(n+1 / 2) \hbar \omega.\right)$
(a) Hence the partition function is given by
$$
Z=\sum_{n=0}^{\infty} e^{-n \beta \hbar \omega} .
$$
This is a geometric series whose sum is given by
$$
Z=\frac{1}{1-e^{-\beta \hbar \omega}} .
$$
Hence the free energy is given by
$$
F=-k_B T \ln Z=k_B T \ln \left[1-e^{-\beta \hbar \omega}\right] .
$$
(b) The entropy is given by
$$
S=-\frac{\partial F}{\partial T}=\frac{\hbar \omega}{T} \frac{1}{e^{\beta \hbar \omega}-1}-\ln \left[1-e^{-\beta \hbar \omega}\right] .
$$
Consider a system in thermal contact with a reservoir at temperature $T$. Show that the mean square fluctuation in the energy of the system is given by
$$
(\Delta E)^2 \equiv\left\langle(E-\langle E\rangle)^2\right\rangle=\left\langle E^2\right\rangle-\langle E\rangle^2=k_B T^2\left(\frac{\partial U}{\partial T}\right)_V,
$$
where $U$ means the average energy, i.e. $\langle E\rangle$.
Hint: It is simplest to start off with an expression for $U$ involving sums over states, and show that, differentiating this with respect to $T$, the right hand side of Eq. (1) gives the energy fluctuation.
We have
$$
U \equiv\langle E\rangle=\frac{1}{Z} \sum_n E_n e^{-\beta E_n},
$$
and
$$
\left\langle E^2\right\rangle=\frac{1}{Z} \sum_n E_n^2 e^{-\beta E_n},
$$
where
$$
Z=\sum_n e^{-\beta E_n} .
$$
Differentiating Eq. (2) with respect to $T$ gives (remember $Z$ also depends on $T$ )
$$
\begin{aligned}
\frac{\partial U}{\partial T} & =\frac{1}{k_B T^2}\left[\frac{1}{Z} \sum_n E_n^2 e^{-\beta E_n}-\frac{1}{Z^2}\left(\sum_n E_n e^{-\beta E_n}\right) \sum_m\left(E_m e^{-\beta E_m}\right)\right] \
& =\frac{1}{k_B T^2}\left(\left\langle E^2\right\rangle-\langle E\rangle^2\right) \
& =\frac{1}{k_B T^2}\left\langle(E-\langle E\rangle)^2\right\rangle .
\end{aligned}
$$
Note: This shows that the mean square fluctuation in the energy is proportional to $\partial U / \partial T$, the heat capacity (also called the specific heat). Since $U$, and hence the specific heat, is extensive the root mean square fluctuation in the energy (which is a typical deviation of the energy from its mean) is proportional to $\sqrt{N}$. We have seen several specific examples of $\sqrt{N}$ fluctuations in the course, and here we see that the fluctuations in the energy vary quite generally in this way.
Diatomic molecules can rotate which gives a contribution to the energy in addition to that from translational motion. According to quantum mechanics the energy is quantized and given by
$$
\epsilon_j=j(j+1) \epsilon_0
$$
where $j=0,1,2, \cdots$ is the angular momentum quantum number, and $\epsilon_0$ is related to the moment of inertia and Planck’s constant. The multiplicity (degeneracy) of each level is $2 j+1$.
(a) Write down an expression for the partition function as a sum over $j$.
Note: It is important to understand that the sum in the partition function is over all states, not energies, and the degeneracy matters.
(b) Evaluate the partition function approximately for high temperatures, $k_B T \gg \epsilon_0$, by replacing the sum by an integral.
(c) Evaluate the partition function approximately for low temperatures, $k_B T \ll \epsilon_0$, by just considering the first two terms in the sum.
(d) Determine the energy $U$ and heat capacity $C$ in these two limits.
Note: You should find that $C$ approaches $k_B$ at high temperatures (unity in Kittel’s units).
(e) Sketch the behavior of $U$ and $C$ as a function of temperature, indicating the limiting behaviors for $T \rightarrow 0$ and $T \rightarrow \infty$.
The energy levels of a diatomic molecule coming from its rotation motion are given by
$$
\epsilon(j)=j(j+1) \epsilon_0
$$
$j=0,1,2, \cdots$, and their degeneracy (multiplicity) is $2 j+1$.
(a) Hence the partition function is given by
$$
Z=\sum_{j=0}^{\infty}(2 j+1) e^{-\beta j(j+1) \epsilon_0},
$$
(note the inclusion of the degeneracy factor $2 j+1$ ). While this cannot be evaluated exactly in closed form, it can be evaluated for very low $T$ and very high $T$.
(b) We first consider very high $T$, for which the Boltzmann factor $e^{-\beta j(j+1) \epsilon_0}$ doesn’t change much from one $j$ to the next, and so we can replace the sum by an integral:
$$
Z \simeq \int_0^{\infty}(2 j+1) e^{-\beta j(j+1) \epsilon_0} d j=-\frac{1}{\beta \epsilon_0}\left[e^{-\beta j(j+1) \epsilon_0}\right]_0^{\infty}=\frac{1}{\beta \epsilon_0} .
$$
(c) In the low $T$ limit we just consider the first two terms (the rest will be negligible) so
$$
Z \simeq 1+3 e^{-\beta 2 \epsilon_0} .
$$
(d) Now $U=-(\partial / \partial \beta) \ln Z$ and applying this to Eq. (4) gives
$$
U=\frac{\partial}{\partial \beta} \ln \left(\beta \epsilon_0\right)=\frac{1}{\beta}=k_B T .
$$
The specific heat is
$$
C=\frac{\partial U}{\partial T}=k_B .
$$
We see that the specific heat tends to a constant at high temperature.
At low temperature, we obtain from Eq. (5)
$$
U=-\frac{\partial}{\partial \beta} \ln \left[1+3 e^{-2 \beta \epsilon_0}\right]=\frac{6 \epsilon_0 e^{-2 \beta \epsilon_0}}{1+3 e^{-2 \beta \epsilon_0}} \simeq 6 \epsilon_0 e^{-2 \beta \epsilon_0} .
$$
The specific heat is given by
$$
C=\frac{\partial U}{\partial T}=12 k_B\left(\frac{\epsilon_0}{k_B T}\right)^2 e^{-2 \beta \epsilon_0} .
$$
Both the energy and specific heat vanish exponentially for $T \rightarrow 0$.
(e) Sketching the energy and specific heat turns out to be a bit tricky. For example you may imagine that the specific heat increases monotonically from 0 to $k_B$ as $T$ increases from 0 to $\infty$, but it actually overshoots, with a peak value of about $1.1 k_B$ at $k_B T \simeq 0.81 \epsilon_0$. Since this is not obvious, you will get full credit for a monotonically increasing curve. Also, the energy actually tends to $k_B T-\epsilon_0 / 3$ at high $T$ (the constant was not given at our level of approximation) and so it actually lies parallel to the line $U=k_B T$ at high $T$ but not on it. However, if you drew the curve equaling $k_B T$ at high $T$ you will get full credit.
The figure below is the correct result obtained numerically. The solid line is for $U$ and the dashed line for $C$. The dotted lines indicate the limits of $C / k_B$ and $U / \epsilon_0$ at high temperature ( 1 and $k_B T / \epsilon_0-1 / 3$ respectively).
MY-ASSIGNMENTEXPERT™可以为您提供 sydney AMME2262 Thermodynamics热力学课程的代写代考和辅导服务!