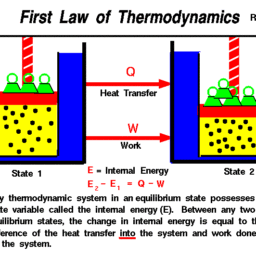
MY-ASSIGNMENTEXPERT™可以为您提供ntnu.edu TEP4120 Thermodynamics热力学课程的代写代考和辅导服务!
这是挪威科技大学 热力学课程的代写成功案例。
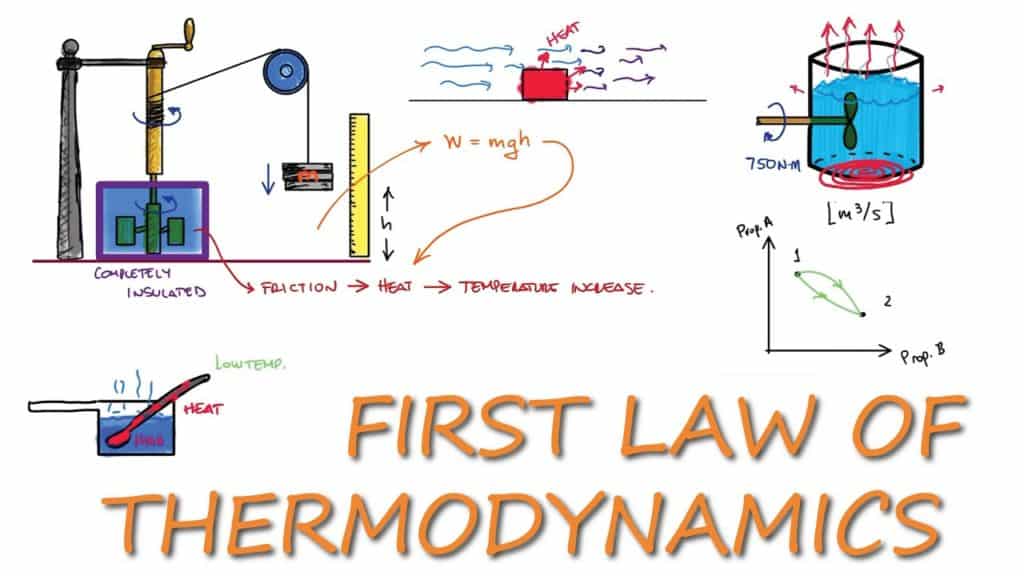
TEP4120课程简介
Course content
Concepts and definitions; the thermodynamic system, properties, phase equilibrium of pure substances, equations of state for gases, tables of thermodynamic properties, work and heat. First law of thermodynamics; thermodynamic cycles, change of state, internal energy, enthalpy, specific heat; open systems, steady-state and transient processes. Second law of thermodynamics; reversible and irreversible processes, the Carnot cycle, the thermodynamic temperature scale, entropy, the entropy production concept. Thermodynamic power cycles, refrigeration cycles, the Otto cycle and the Diesel cycle, the gas-turbine process. Introduction to exergy analysis.
Prerequisites
LKnowledge: The course provides the student with knowledge about: – Conservation laws for mass and energy (including the 1st law of Thermodynamics). – Forms of energy such as work (power) and heat, internal energy and enthalpy. – Entropy and the 2nd law of thermodynamics. – Reasons for thermodynamic losses in the form of irreversibilities. – The quality of different forms of energy measured as the ability to produce work. – Destruction of energy quality in processes. – Ideal gas model, its assumptions, applications and limitations. – Different cyclic processes such as Carnot, Rankine, Otto, Diesel and Brayton. The course gives the student insight about: – Operation of steam and gas based power stations, internal combustion engines, heat pumps and refrigeration cycles. – The main components of heat & power processes, such as steam and gas turbines, compressors, pumps, fans, heat exchangers and valves. – The ability of fluids to change phase (solid, liquid and gas).
TEP4120 Thermodynamics HELP(EXAM HELP, ONLINE TUTOR)
Compare the changes in specific internal energy and enthalpy for liquid water that is heated from a temperature of $20^{\circ} \mathrm{C}$ to $60^{\circ} \mathrm{C}$ at a constant pressure of $100 \mathrm{kPa}$ as determined using the following approaches: a) saturated liquid values, b) constant specific heat evaluated at the average temperature, c) constant specific heat evaluated at the initial temperature.
Consider a constant volume process involving heat addition to a closed system consisting of an ideal gas with no changes in kinetic or potential energy. Is the required heat transfer for raising the temperature from 295 to $305 \mathrm{~K}$ the same as the heat transfer required from 345 to $355 \mathrm{~K}$ ? If not, then what assumption would be needed for them to be the same? Explain.
A fixed mass of an ideal gas is heated from 40 to $60^{\circ} \mathrm{C}$ at a constant pressure of (a) $100 \mathrm{kPa}$ and (b) $300 \mathrm{kPa}$. For which case do you think the energy required will be greater? Use thermodynamic relations to explain. Do not consider any changes in kinetic and potential energy.
A fixed mass of an ideal gas is heated from 40 to $60^{\circ} \mathrm{C}$ (a) at constant volume and (b) at constant pressure. For which case do you think the energy required will be greater? Use thermodynamic relations to explain.
Compare the internal energy change of air (treated as an ideal gas) that is heated from $300 \mathrm{~K}$ to $400 \mathrm{~K}$ as determined using 3 different approaches:
a) ideal gas tables for air,
b) constant specific heat for air evaluated at the average temperature,
c) constant specific heat for air evaluated at the initial temperature.
A balloon filled with air has been sitting outside in an environment at $0^{\circ} \mathrm{C}$ and then is moved indoors where the temperature is $20^{\circ} \mathrm{C}$. The balloon comes into thermal equilibrium with the room and has a final volume that is $3 \%$ larger than the initial volume. The initial pressure and volume of the air within the balloon are $125 \mathrm{kPa}$ and $0.11 \mathrm{~m}^3$, respectively. Assume that the balloon material properties are such that the pressure and volume of the air within the balloon are related according to $\mathrm{P}=\mathrm{aV}^{\mathrm{b}}$, where $\mathrm{a}$ and $\mathrm{b}$ are empirical constants. You can also assume constant specific heat. Determine the heat transfer to the balloon during the process, in $\mathrm{J}$ ?
MY-ASSIGNMENTEXPERT™可以为您提供CATALOG.WINONA.EDU PHYS451 QUANTUM MECHANICS量子力学课程的代写代考和辅导服务