如果你也在 怎样代写有机化学organic chemistry这个学科遇到相关的难题,请随时右上角联系我们的24/7代写客服。有机化学organic chemistry是化学的一个分支,研究含有碳-碳共价键的有机化合物的结构、性质和反应。对性质的研究包括物理和化学性质,以及对化学反应性的评估,以了解其行为。有机反应的研究包括天然产品、药物和聚合物的化学合成,以及在实验室和通过理论(in silico)研究单个有机分子。
有机化学organic chemistry研究的化学品范围包括碳氢化合物(只含碳和氢的化合物)以及以碳为基础但也含有其他元素的化合物,特别是氧、氮、硫、磷(包括在许多生化制品中)和卤素。有机金属化学是研究含有碳-金属键的化合物。此外,当代研究的重点是涉及其他有机金属的有机化学,包括镧系元素,但特别是过渡金属锌、铜、钯、镍、钴、钛和铬。
my-assignmentexpert™ 有机化学organic chemistry作业代写,免费提交作业要求, 满意后付款,成绩80\%以下全额退款,安全省心无顾虑。专业硕 博写手团队,所有订单可靠准时,保证 100% 原创。my-assignmentexpert™, 最高质量的有机化学organic chemistry作业代写,服务覆盖北美、欧洲、澳洲等 国家。 在代写价格方面,考虑到同学们的经济条件,在保障代写质量的前提下,我们为客户提供最合理的价格。 由于统计Statistics作业种类很多,同时其中的大部分作业在字数上都没有具体要求,因此有机化学organic chemistry作业代写的价格不固定。通常在经济学专家查看完作业要求之后会给出报价。作业难度和截止日期对价格也有很大的影响。
想知道您作业确定的价格吗? 免费下单以相关学科的专家能了解具体的要求之后在1-3个小时就提出价格。专家的 报价比上列的价格能便宜好几倍。
my-assignmentexpert™ 为您的留学生涯保驾护航 在化学Chemical作业代写方面已经树立了自己的口碑, 保证靠谱, 高质且原创的化学Chemical代写服务。我们的专家在有机化学organic chemistry代写方面经验极为丰富,各种有机化学organic chemistry相关的作业也就用不着 说。
我们提供的有机化学organic chemistry及其相关学科的代写,服务范围广, 其中包括但不限于:
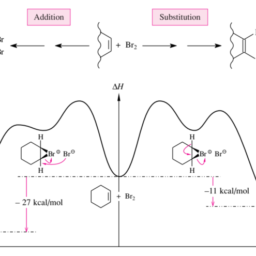
化学代写|有机化学代写organic chemistry代考|Addition of Bromine
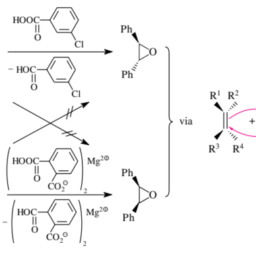
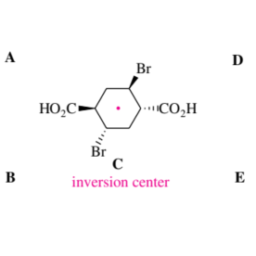
The trans addition of bromine to $\mathrm{C}=\mathrm{C}$ double bonds follows the onium mechanism generally formulated in Figure 3.32. Cyclohexene reacts stereoselectively to give (racemic!) trans-1,2-dibromocyclohexane:
trans-Selectivity is also exhibited by the additions of bromine to fumaric or maleic acid, which follow the same mechanism:
This is proven by the fact that the reaction with fumaric acid gives mesodibromosuccinic acid, whereas maleic acid gives the chiral dibromosuccinic acid, of course as a racemic mixture. Note that these reactions are stereospecific.
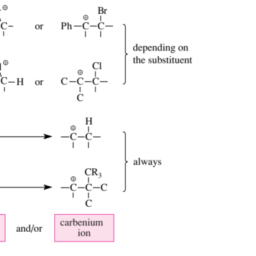
Instead of a bromonium ion, in certain cases an isomeric acyclic and sufficiently stable cation can occur as an intermediate of the bromine addition to olefins. This holds true for bromine-containing benzyl cations. Therefore, the bromine addition to $\beta$ methyl styrene shown in Figures $3.5$ and $3.6$ takes place without stereocontrol.
The addition of $\mathrm{Cl}{2}$ to $\mathrm{C}=\mathrm{C}$ double bonds is trans-selective only when it takes place via three-membered chloronium ions. But it can also take place without stereocontrol, namely, when carbenium ion intermediates appear instead of chloronium ions. This is observed in $\mathrm{Cl}{2}$ additions, which can take place via benzyl or tert-alkyl cations.
In general, an addition of $\mathrm{I}_{2}$ to $\mathrm{C}=\mathrm{C}$ double bonds is thermodynamically impossible, although an iodonium ion can still form.
化学代写|有机化学代写organic chemistry代考|The Formation of Halohydrins; Halolactonization and Haloetherification
Halohydrins are $\beta$-halogenated alcohols. They can be obtained in $\mathrm{H}{2} \mathrm{O}$-containing solvents from olefins and reagents, which transfer $\mathrm{Hal}^{+}$ions. $N$-Bromosuccinimide (transfers $\mathrm{Br}^{+}$; Figures $3.33$ and $3.34$ as well as $3.36$ ), chloramine- $\mathrm{T}$ (transfers $\mathrm{Cl}^{+}$; Figure $3.35$ ), and elemental iodine (transfers $\mathrm{I}^{+}$; Figure 3.36) have this ability. Bromonium and chloronium ions are then attacked by $\mathrm{H}{2} \mathrm{O}$ according to an $\mathrm{S}{\mathrm{N}} 2$ mechanism. This furnishes the protonated bromo- or chlorohydrins, which are subsequently deprotonated. Instead of $\mathrm{H}{2} \mathrm{O}, \mathrm{COOH}$ or $\mathrm{OH}$ groups of the substrate located at a suitable distance can also open the halonium ion intermediate through a nucleophilic backside attack. In this way, cyclic halohydrin derivatives are produced (Figure 3.36). They are referred to as halolactones or haloethers.
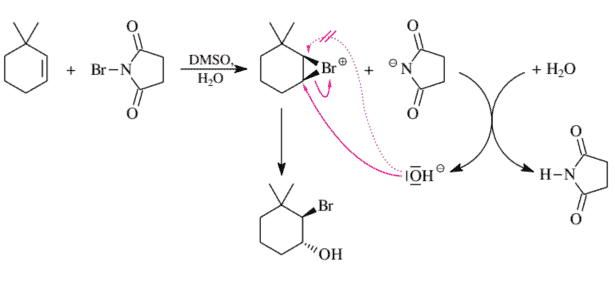
With NBS in aqueous DMSO, cyclohexene gives racemic trans-2-bromo-1-cyclohexanol. This stereochemical result means that we have a trans addition. In the analogous bromohydrin formation from 3,3-dimethylcyclohexene, the analogous dimethylated 2-bromo-1-cyclohexanol is also produced trans-selectively as well as regioselectively (Figure 3.33). In the bromonium ion intermediate the backside attack by the $\mathrm{H}{2} \mathrm{O} \mathrm{mol-}$ ecule does not take place at the hindered neopentyl center according to the rules for $S{N}$ 2 reactivity (Section 2.4.4).
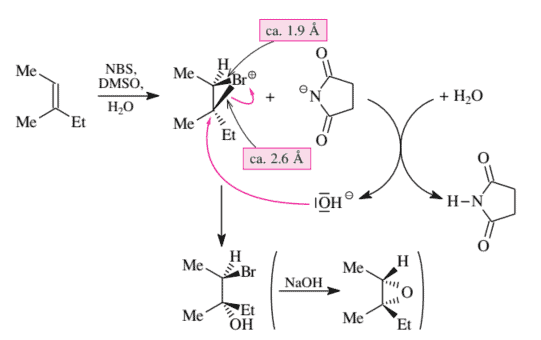
As shown in Figure 3.34, trisubstituted olefins are also converted to bromohydrins by NBS in an aqueous organic solvent. This reaction takes place via a trans addition and therefore must take place via a bromonium ion. Once again, the reaction is also regioselective. At first glance, the regioselectivity of this reaction might seem surprising: The $\mathrm{H}{2} \mathrm{O}$ molecule attacks the bromonium ion intermediate at the tertiary instead of at the secondary $\mathrm{C}$ atom. One might have expected the backside $\left(\mathrm{S}{\mathrm{N}} 2\right)$ attack of the nucleophile to be directed at the secondary $\mathrm{C}$ atom of the bromonium ion (cf. Section 2.4.4). However, this is not the case. The bromonium ion is very distorted: The $\mathrm{C}{\text {sec }}-\mathrm{Br}^{+}$bond at $1.9 \AA$ is considerably shorter than and consequently considerably stronger than the $\mathrm{C}{\text {ter }}-\mathrm{Br}^{+}$bond. The latter is stretched to $2.6 \AA$ and thereby weakened. This distortion reduces the ring strain of the bromonium ion. The distortion becomes possible because the stretching of the $\mathrm{C}_{\text {tert }}-\mathrm{Br}^{+}$bond produces a partial positive charge on the tertiary $\mathrm{C}$ atom, which is stabilized by the alkyl substituents located there. In this bromonium ion, the bromine atom has almost separated from the tertiary ring $\mathrm{C}$ atom (but only almost, because otherwise the result would not be $100 \%$ trans addition).
化学代写|有机化学代写ORGANIC CHEMISTRY代考|Solvomercuration of Olefins: Hydration of C=C Double Bonds through Subsequent Reduction
Mercury(II) salts add to $\mathrm{C}=\mathrm{C}$ double bonds (Figure 3.37) in nucleophilic solvents according to the onium mechanism of Figure 3.32. However, the heterocyclic primary product is not called an onium, but rather a mercurinium ion. Its ring opening in an $\mathrm{H}{2} \mathrm{O}$-containing solvent gives a trans-configured alcohol, which can then be demercurated with $\mathrm{NaBH}{4}$. The hydration product of the original olefin is obtained and is, in our case, cyclohexanol.
The mechanism of this defunctionalization was discussed in connection with Figure 1.12. It took place via approximately planar radical intermediates. This is why in the reduction of alkyl mercury(II) acetates, the $\mathrm{C}-\mathrm{Hg}$ bond changes to the $\mathrm{C}-\mathrm{H}$ bond without stereocontrol. The stereochemical integrity of the mercury-bearing stereocenter is thus lost. When the mercurated alcohol in Figure $3.37$ is reduced with $\mathrm{NaBD}{4}$ rather than $\mathrm{NaBH}{4}$, the deuterated cyclohexanol is therefore produced as a mixture of diastereomers. Asymmetrically substituted $\mathrm{C}=\mathrm{C}$ double bonds are hydrated according to the same mechanism (Figure 3.38). The regioselectivity is high, and the explanation for this is that the mercurinium ion intermediate is distorted in the same way as the bromonium ion in Figure 3.34. The $\mathrm{H}{2} \mathrm{O}$ preferentially breaks the stretched and therefore weakened $\mathrm{C}{\text {sec }}-\mathrm{Hg}^{+}$bond by a backside attack and does not affect the shorter and therefore more stable $\mathrm{C}_{\text {prim }}-\mathrm{Hg}^{+}$bond.
From the $\mathrm{Hg}$-containing alcohol in Figure $3.38$ and $\mathrm{NaBH}{4}$ one can obtain the $\mathrm{Hg}$ free alcohol. The overall result is a hydration of the $\mathrm{C}=\mathrm{C}$ double bond. According to the nomenclature of Section 3.3.3, its regioselectivity corresponds to a Markovnikov addition. It is complementary to the regioselectivity of the reaction sequence hydroboration/oxidation/hydrolysis (Table 3.1). The latter sequence would have converted the same olefin regioselectively into the primary instead of the secondary alcohol. Besides $\mathrm{H}{2} \mathrm{O}$, simple alcohols or acetic acid can also be added to olefins by solvomercuration/reduction. Figure $3.39$ shows MeOH addition as an example. The regioselectivities of this reaction and of the $\mathrm{H}_{2} \mathrm{O}$ addition in Figure $3.38$ are identical.
有机化学代写
化学代写|有机化学代写ORGANIC CHEMISTRY代考|ADDITION OF BROMINE
溴的反式加成C=C双键遵循图 3.32 中通常制定的鎓机制。环己烯立体选择性反应得到r一种C和米一世C!trans-1,2-二溴环己烷:
在富马酸或马来酸中添加溴也表现出反式选择性,其遵循相同的机制:
与富马酸反应产生内二溴琥珀酸,而马来酸产生手性二溴琥珀酸,当然作为外消旋混合物,这一事实证明了这一点。请注意,这些反应是立体特异性的。
代替溴离子,在某些情况下,异构的无环且足够稳定的阳离子可以作为溴加成烯烃的中间体出现。这适用于含溴的苄基阳离子。因此,溴的加入b甲基苯乙烯如图所示3.5和3.6在没有立体控制的情况下进行。
增加$\mathrm{Cl}{2}$ to $\mathrm{C}=\mathrm{C}$ double bonds is trans-selective only when it takes place via three-membered chloronium ions. But it can also take place without stereocontrol, namely, when carbenium ion intermediates appear instead of chloronium ions. This is observed in $\mathrm{Cl}{2}$加成,可以通过苄基或叔烷基阳离子发生。
一般来说,添加一世2到C=C尽管仍然可以形成碘鎓离子,但双键在热力学上是不可能的。
化学代写|有机化学代写ORGANIC CHEMISTRY代考|THE FORMATION OF HALOHYDRINS; HALOLACTONIZATION AND HALOETHERIFICATION
卤代醇是b-卤代醇。它们可以在$\beta$-halogenated alcohols. They can be obtained in $\mathrm{H}{2} \mathrm{O}$-containing solvents from olefins and reagents, which transfer $\mathrm{Hal}^{+}$ions. $N$-Bromosuccinimide (transfers $\mathrm{Br}^{+}$; Figures $3.33$ and $3.34$ as well as $3.36$ ), chloramine- $\mathrm{T}$ (transfers $\mathrm{Cl}^{+}$; Figure $3.35$ ), and elemental iodine (transfers $\mathrm{I}^{+}$; Figure 3.36) have this ability. Bromonium and chloronium ions are then attacked by $\mathrm{H}{2} \mathrm{O}$ according to an $\mathrm{S}{\mathrm{N}} 2$ mechanism. This furnishes the protonated bromo- or chlorohydrins, which are subsequently deprotonated. Instead of $\mathrm{H}{2} \mathrm{O}, \mathrm{COOH}$ or $\mathrm{OH}$基团也可以通过亲核背面攻击打开卤离子中间体。这样就产生了环状卤代醇衍生物F一世G在r和3.36. 它们被称为卤代内酯或卤代醚。
使用 NBS 在 DMSO 水溶液中,环己烯得到外消旋的 trans-2-bromo-1-cyclohexanol。这种立体化学结果意味着我们有反式加成。在由 3,3-二甲基环己烯形成类似的溴醇时,类似的二甲基化 2-溴-1-环己醇也可以反式选择性和区域选择性生成F一世G在r和3.33. 在溴离子中间受到 $\mathrm{H} {2} \mathrm{O} \mathrm{mol-}的背面攻击和C在l和d这和sn这吨吨一种ķ和pl一种C和一种吨吨H和H一世nd和r和dn和这p和n吨是lC和n吨和r一种CC这rd一世nG吨这吨H和r在l和sF这rS {N}$ 2 反应性小号和C吨一世这n2.4.4.
如图 3.34 所示,三取代烯烃也可通过 NBS 在水性有机溶剂中转化为溴代醇。该反应通过反式加成发生,因此必须通过溴离子发生。再一次,该反应也是区域选择性的。乍一看,这个反应的区域选择性似乎令人惊讶:$\mathrm{H}{2} \mathrm{O}$ molecule attacks the bromonium ion intermediate at the tertiary instead of at the secondary $\mathrm{C}$ atom. One might have expected the backside $\left(\mathrm{S}{\mathrm{N}} 2\right)$ attack of the nucleophile to be directed at the secondary $\mathrm{C}$ atom of the bromonium ion (cf. Section 2.4.4). However, this is not the case. The bromonium ion is very distorted: The $\mathrm{C}{\text {sec }}-\mathrm{Br}^{+}$bond at $1.9 \AA$ is considerably shorter than and consequently considerably stronger than the $\mathrm{C}{\text {ter }}-\mathrm{Br}^{+}$bond. The latter is stretched to $2.6 \AA$ and thereby weakened. This distortion reduces the ring strain of the bromonium ion. The distortion becomes possible because the stretching of the $\mathrm{C}_{\text {tert }}-\mathrm{Br}^{+}$bond produces a partial positive charge on the tertiary $\mathrm{C}$ atom, which is stabilized by the alkyl substituents located there. In this bromonium ion, the bromine atom has almost separated from the tertiary ring $\mathrm{C}$ atom (but only almost, because otherwise the result would not be $100 \%$反式加法)。
化学代写|有机化学代写ORGANIC CHEMISTRY代考|SOLVOMERCURATION OF OLEFINS: HYDRATION OF C=C DOUBLE BONDS THROUGH SUBSEQUENT REDUCTION
汞一世一世盐添加到C=C双键F一世G在r和3.37根据图 3.32 的鎓机理,在亲核溶剂中。但是,杂环初级产物不称为鎓,而是称为汞离子。它以 $\mathrm{H} {2} \mathrm{O}开环−C这n吨一种一世n一世nGs这l在和n吨G一世在和s一种吨r一种ns−C这nF一世G在r和d一种lC这H这l,在H一世CHC一种n吨H和nb和d和米和rC在r一种吨和d在一世吨H\mathrm{NaBH} {4}$。得到原始烯烃的水合产物,在我们的例子中是环己醇。
结合图讨论了这种去功能化的机制1.12. 它通过近似平面的自由基中间体发生。这就是为什么在还原烷基汞一世一世醋酸盐C−HG债券的变化C−H没有立体控制的结合。含汞立体中心的立体化学完整性因此丧失。当图中的汞醇3.37用 $\mathrm{C}-\mathrm{Hg}$ bond changes to the $\mathrm{C}-\mathrm{H}$ bond without stereocontrol. The stereochemical integrity of the mercury-bearing stereocenter is thus lost. When the mercurated alcohol in Figure $3.37$ is reduced with $\mathrm{NaBD}{4}$ rather than $\mathrm{NaBH}{4}$, the deuterated cyclohexanol is therefore produced as a mixture of diastereomers. Asymmetrically substituted $\mathrm{C}=\mathrm{C}$ double bonds are hydrated according to the same mechanism (Figure 3.38). The regioselectivity is high, and the explanation for this is that the mercurinium ion intermediate is distorted in the same way as the bromonium ion in Figure 3.34. The $\mathrm{H}{2} \mathrm{O}$ preferentially breaks the stretched and therefore weakened $\mathrm{C}{\text {sec }}-\mathrm{Hg}^{+}$bond by a backside attack and does not affect the shorter and therefore more stable $\mathrm{C}_{\text {prim }}-\mathrm{Hg}^{+}$键。
来自HG图中含酒精3.38$\mathrm{Hg}$-containing alcohol in Figure $3.38$ and $\mathrm{NaBH}{4}$ one can obtain the $\mathrm{Hg}$ free alcohol. The overall result is a hydration of the $\mathrm{C}=\mathrm{C}$ double bond. According to the nomenclature of Section 3.3.3, its regioselectivity corresponds to a Markovnikov addition. It is complementary to the regioselectivity of the reaction sequence hydroboration/oxidation/hydrolysis (Table 3.1). The latter sequence would have converted the same olefin regioselectively into the primary instead of the secondary alcohol. Besides $\mathrm{H}{2} \mathrm{O}$, simple alcohols or acetic acid can also be added to olefins by solvomercuration/reduction. Figure $3.39$ shows MeOH addition as an example. The regioselectivities of this reaction and of the $\mathrm{H}_{2} \mathrm{O}$ addition in Figure $3.38$ 美元是相同的。
化学代写|有机化学代写organic chemistry代考 请认准UprivateTA™. UprivateTA™为您的留学生涯保驾护航。
电磁学代考
物理代考服务:
物理Physics考试代考、留学生物理online exam代考、电磁学代考、热力学代考、相对论代考、电动力学代考、电磁学代考、分析力学代考、澳洲物理代考、北美物理考试代考、美国留学生物理final exam代考、加拿大物理midterm代考、澳洲物理online exam代考、英国物理online quiz代考等。
光学代考
光学(Optics),是物理学的分支,主要是研究光的现象、性质与应用,包括光与物质之间的相互作用、光学仪器的制作。光学通常研究红外线、紫外线及可见光的物理行为。因为光是电磁波,其它形式的电磁辐射,例如X射线、微波、电磁辐射及无线电波等等也具有类似光的特性。
大多数常见的光学现象都可以用经典电动力学理论来说明。但是,通常这全套理论很难实际应用,必需先假定简单模型。几何光学的模型最为容易使用。
相对论代考
上至高压线,下至发电机,只要用到电的地方就有相对论效应存在!相对论是关于时空和引力的理论,主要由爱因斯坦创立,相对论的提出给物理学带来了革命性的变化,被誉为现代物理性最伟大的基础理论。
流体力学代考
流体力学是力学的一个分支。 主要研究在各种力的作用下流体本身的状态,以及流体和固体壁面、流体和流体之间、流体与其他运动形态之间的相互作用的力学分支。
随机过程代写
随机过程,是依赖于参数的一组随机变量的全体,参数通常是时间。 随机变量是随机现象的数量表现,其取值随着偶然因素的影响而改变。 例如,某商店在从时间t0到时间tK这段时间内接待顾客的人数,就是依赖于时间t的一组随机变量,即随机过程
Matlab代写
MATLAB 是一种用于技术计算的高性能语言。它将计算、可视化和编程集成在一个易于使用的环境中,其中问题和解决方案以熟悉的数学符号表示。典型用途包括:数学和计算算法开发建模、仿真和原型制作数据分析、探索和可视化科学和工程图形应用程序开发,包括图形用户界面构建MATLAB 是一个交互式系统,其基本数据元素是一个不需要维度的数组。这使您可以解决许多技术计算问题,尤其是那些具有矩阵和向量公式的问题,而只需用 C 或 Fortran 等标量非交互式语言编写程序所需的时间的一小部分。MATLAB 名称代表矩阵实验室。MATLAB 最初的编写目的是提供对由 LINPACK 和 EISPACK 项目开发的矩阵软件的轻松访问,这两个项目共同代表了矩阵计算软件的最新技术。MATLAB 经过多年的发展,得到了许多用户的投入。在大学环境中,它是数学、工程和科学入门和高级课程的标准教学工具。在工业领域,MATLAB 是高效研究、开发和分析的首选工具。MATLAB 具有一系列称为工具箱的特定于应用程序的解决方案。对于大多数 MATLAB 用户来说非常重要,工具箱允许您学习和应用专业技术。工具箱是 MATLAB 函数(M 文件)的综合集合,可扩展 MATLAB 环境以解决特定类别的问题。可用工具箱的领域包括信号处理、控制系统、神经网络、模糊逻辑、小波、仿真等。